
Now with a rapidly aging population, the treatment of age-related diseases is increasingly becoming a major issue. Parkinson's disease, which prevents the patients from controlling their body movements freely, mostly appears in people aged 50's and 60's. It is one of the intractable diseases which we have no fundamental cure for. Parkinson's disease is considered to be caused by degeneration of dopamine-producing neuronal cells for some cause, so expectations for the regenerative medicine that will enable us to replace lost dopaminergic neuronal cells with graft ones have increased these years. Some stem cells such as ES cells (embryonic-stem-cells), iPS cells (induced pluripotent stem cells) are supposed to be promising donor cells, and some techniques of effectively inducing the differentiation into dopaminergic cells have been developed. However, little is known of the suitable differentiation state which keeps cells safest (without tumorigenic transformation of the grafts) and produces large treatment effects for Parkinson's disease (or facilitates the grafts to survive and function successfully).
This time, to closely examine the effectiveness of Parkinson's disease treatment using human ES cells and its side effects, we have prepared two kinds of dopaminergic neuronal cells: ones cultured for a short term to be weakly differentiated and the others cultured for a long term to be highly differentiated. After transplanting them in cynomolgus monkeys in which the symptoms of Parkinson's disease were artificially induced, we determined the differences among them of tumorigenicity and the capacity to produce dopamine by live imaging of the grafts, using MRI and PET scan. The result indicated that any tumor formed by undifferentiated graft cells would be detectable, and highly-differentiated graft cells will function as dopamine-producing cells without being transformed into tumors. Our histopathological investigation confirmed the fact as well. Note that only highly-differentiated graft cells effectively improved the symptom of such disease.
The finding of our research demonstrates that the graft treatment using ES cells may lead to the establishment of the protocol for controlling the differentiation of graft cells and for treating the disease effectively and safely, as well as indicating that molecular imaging techniques such as MRI and PET (Positron Emission Tomography) are exceptionally useful evaluation methods to noninvasively verify the graft treatment. We are going to advance our research to develop the technique of highly precise live imaging of graft cells, and to apply it to human cases.
* This study was a joint program between Institute for Frontier Medical Sciences, Kyoto University and a study group in Center for iPS Cell Research and Application, Kyoto University (researcher Daisuke Doi, associate professor Jun Takahashi) and a team in Functional Probe Research Laboratory, RIKEN Center for Molecular Imaging Science (team leader Hirotaka Onoe, deputy team leader Takuya Hayashi, researcher Toshiyuki Kawasaki). See here for more details of the study (STEM CELLS Vol30-5, 935–945, May 2012).
| Controlled | Parkinson's disease model monkey |
Parkinson's disease model monkey |
|
| PET images detecting dopamine neuronal cells. | Dopaminergic neuronal cells disappeared. | Graft cells produce dopaminergic neuronal cells. | No tumor signals by PET seen in MRI images of graft cells. |
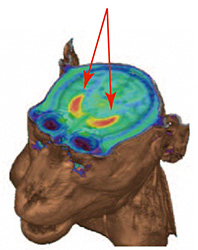 |
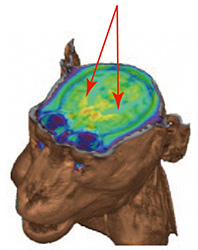 |
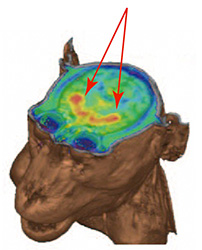 |
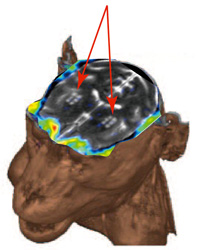 |
| 12 months after transplantation | |||
