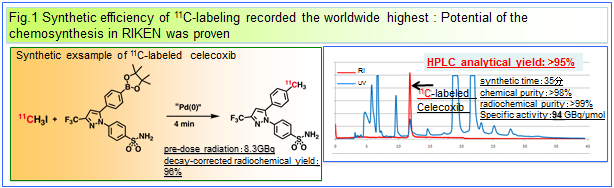
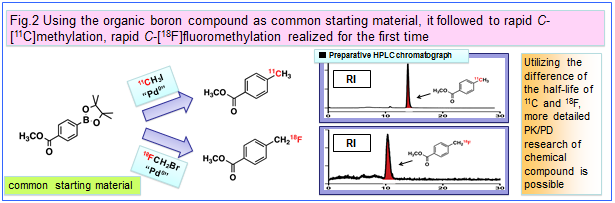
In cooperation with the Molecular Imaging Medicinal Chemistry Laboratory, our laboratory is developing the general synthetic methodology of short-lived PET probes for promotion of PET molecular imaging science. With the objective of applying the potentials of organic chemistry to life science, we seek to realize the new synthetic methodology of PET molecular probes by using organometallic catalysts such as Pd, Rh, and Cu etc.. As one of our main projects, we are striving toward introducing
11C into carbon frameworks of bioactive organic compounds by developing Pd
0-mediated rapid
C-[
11C]methylations, by Prof. Masaaki Suzuki's overview as a head of our chemistry groups. These labeling methods provides groundbreaking synthesis for introducing the [
11C]methyl group as the minimum carbon substituent into a carbon framework in the very short time of 5 minutes. We are currently expanding and evolving these rapid methylations from introduction of [
11C]methyl group to [
18F]fluoromethyl group, and furthermore developing the stoichiometry-focused
18F-labeling of oligonucleotides and peptides by the application of Click chemistry.
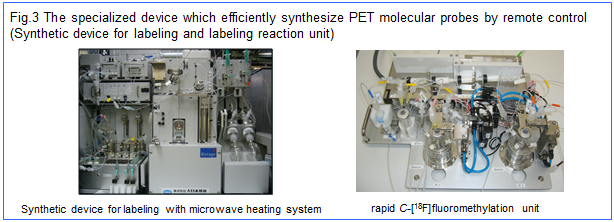
In the field of PET radiolabeling, the performance of the remote-controlled PET probe synthesizer is of key importance for realizing high quality and high yield in the synthesis of PET molecular probes. In this regard, we are also engaged in developing original PET probe synthesizers from the perspectives of not only safety and quick operation emphasized in labeling chemistry but also introducing advantages of experimental techniques ordinarily-used in organic chemistry. Thus, we seek to realize high quality RIKEN original molecular probes and establish the probe library through developing the new labeling methodology from the perspective of synthetically ideals and efficiencies based on organic chemistry.
[Contact information for research-related enquiries]
Hisashi Doi TEL 078-304-7130 Mail
[Contact information for non-research-related enquiries]
Yasuko Kawai TEL 078-304-7130 Mail






